From a STAT opinion piece by Juliana M. Reed, executive director of the Biosimilars Forum:
“Biologic medicines are some of the most important drugs in the world. They are also among the most expensive. Biosimilars are FDA-approved treatments that are similar to another biologic medication that has already been approved by the Food and Drug Administration that treat cancer, diabetes, psoriasis, arthritis, eye disease, and much more. Each biosimilar requires hundreds of millions of dollars and seven to nine years to develop before it can even go to the FDA for approval. Biosimilars, which cost, on average, 50% less than their brand-name predecessors, can help people afford these important medications.
“Greater use of biosimilars can save the U.S. health care system up to $133 billion by 2025. Yet these savings are being completely left on the table because PBMs have prioritized in their formularies high-cost, high-rebate drugs over lower-cost biosimilars. A recent IQVIA report showed that access to Humira biosimilars could have saved the health care system up to $6 billion in one year had they been given greater inclusion in PBMs’ formularies.” [Emphasis added]
Elsewhere in the article, Reed also notes that some Humira biosimilars cost 85% less than the brand-name drug, but “[t]he three largest PBMs denied patients and their families access to these lower-cost medicines because they wanted to maintain their profits”… and due in large part to this, “less than 4% of people who need this medicine have access to these lower-cost versions.”
In contrast, MedBen Rx offers a biosimilar alternative to Humira that will save clients almost $3.3 million this year. as well as a biosimilar version of the insulin Humalog. And we are exploring additional biosimilars that will help employers and plan members further reduce their spend on high-cost medications.
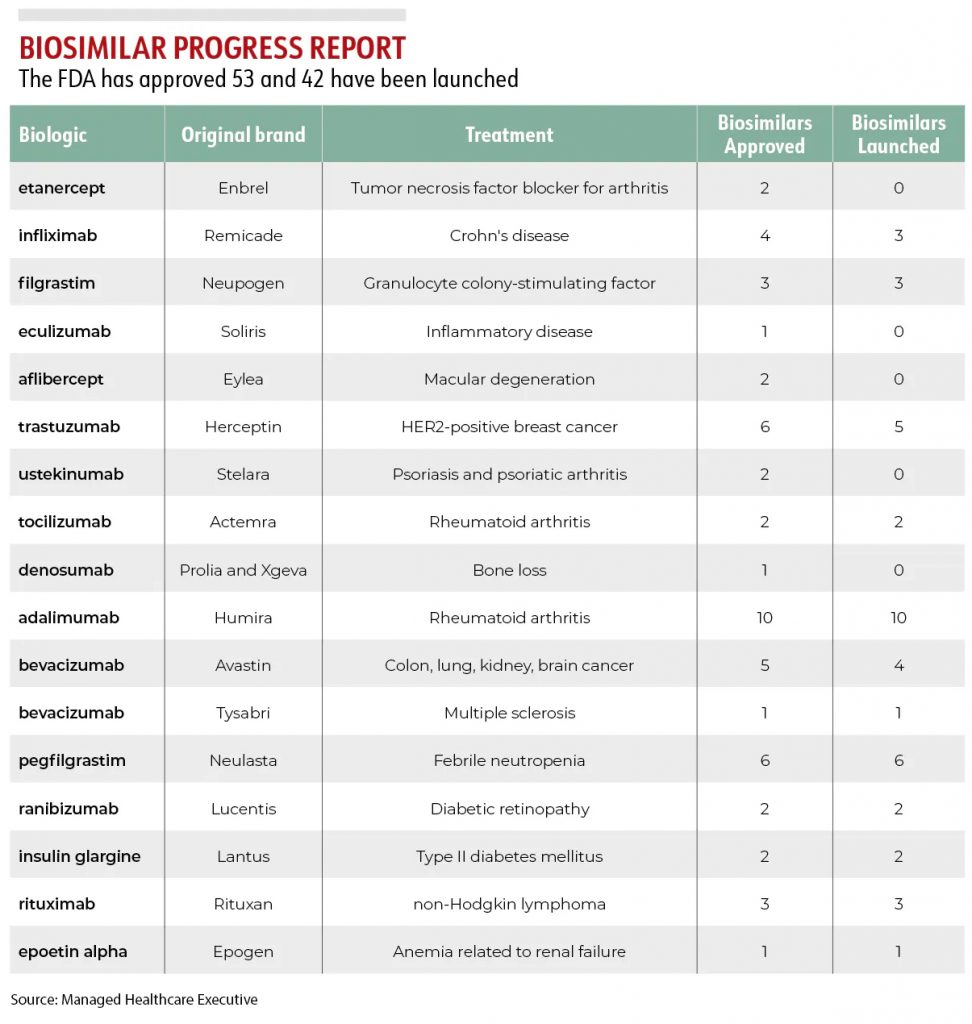
Managed Healthcare Executive has compiled a Progress Report listing every biosimilar currently approved by the FDA.